Chronic Cough in IPF Pathophysiology
Research surrounding the pathophysiology of chronic cough can often be difficult due to the numerous comorbidities that IPF patients experience, resulting in the exact pathophysiology being unknown.1 However, it has been proposed that cough in IPF is likely the result of a chemical or mechanical stimulation of the cough receptors. 2,3
Possible Pathophysiology of Cough in IPF2,3
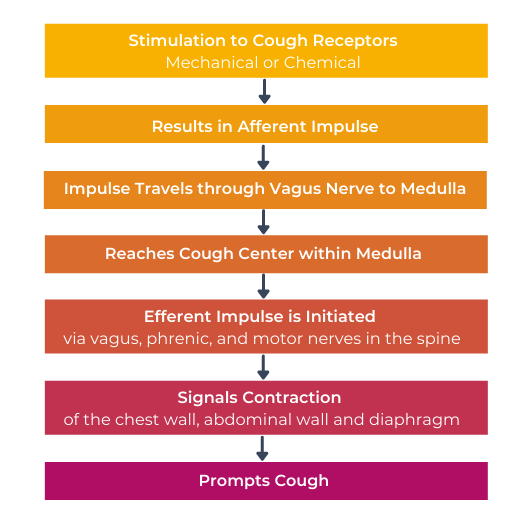
It is thought that IPF patients have an increased cough sensitivity over those without IPF.4,5
This over sensitivity suggests an upregulation of the sensory fibres in the lungs which may lead to chronic coughing as IPF patients are then more prone to minimal levels of stimuli resulting in cough.4,5
Mechanical and Chemical Stimulation
Mechanical and/or chemical stimulation of the cough receptors is not only thought to result in cough itself, but also has the potential to additionally impact IPF patients’.2
Mechanical Stimulation of Cough Receptors
Potentially associated with exacerbating fibrosis leading to architectural distortion.2
Chemical Stimulation of Cough Receptors
Potentially associated with subclinical inflammation.2
The Imbalance of Cough
There is thought to be an imbalance in IPF patients between the stimulation of cough and the suppression response to the stimulation of cough.3
The Imbalance of Cough

There is thought to be an imbalance in IPF patients between the stimulation of cough and the suppression response to the stimulation of cough.3
Role of Opioid Receptors
There is some evidence that the cough reflex sensitivity in patients with IPF is increased, suggesting an upregulation of sensory fibers in the lungs.4,5 Ultimately, the upregulated sensory capability of vagal afferent nerves may lead to chronic cough by reacting to what should normally be imperceptible levels of noxious stimuli.4
Opioid receptors are present throughout the cortex and respiratory tract.6,7 In the presence of inflammation, μ and κ opioid receptors in the respiratory tract are upregulated on peripheral sensory neurons, in association with local production of endogenous opioid ligands by immune cells.8
This activity can reduce local inflammation, and κ agonists have been shown to produce both analgesia and reduced inflammatory activity at multiple points in the inflammatory cascade.9 Multiple endogenous opioids with different relative affinities for receptor subtypes are found in the medullary and pontine respiratory regions, and the mu, kappa, and delta receptors are found in the respiratory-related regions of the brainstem and spinal cord. 10
References
1. Harrison NK., Pulm Pharmacol Ther, 2004. doi: 10.1016/j.pupt.2004.09.029.
2. Ryerson CJ, et al., Respirology, 2011. doi: 10.1111/j.1440-1843.2011.01996.x
3. Van Manen MJ, et al., Eur Respir Rev, 2016. doi: 10.1183/16000617.0090-2015
4. Hope-Gill BD, et al., Am J Respir Crit Care Med, 2003. doi: 10.1164/rccm.200304-597OC
5. Doherty MJ, et al., Thorax, 2000. doi: 10.1136/thorax.55.12.1028
6. Zebraski SE, et al., Life Sci, 2000. doi: 10.1016/s0024-3205(00)00434-3
7. Volkow ND and McLellan AT, N Engl J Med, 2016. doi: 10.1056/NEJMra1507771
8. Stein C, Front Pharmacol, 2013. doi: 10.3389/fphar.2013.00123
9. Albert-Vartanian A, et al., J Clin Pharm Ther, 2016. doi: 10.1111/jcpt.12404
10. Lalley PM, Respir Physiol Neurobiol, 2008. doi: 10.1016/j.resp.2008.02.004

This site is intended for US Healthcare Professionals only.
